
5.85g of NaCl is dissolved in 1L of pure water. The number of ions in
What is the molarity of a solution in which 5.0 g of sodium
What will be the concentration of Cl ions when we mix 1 litre of
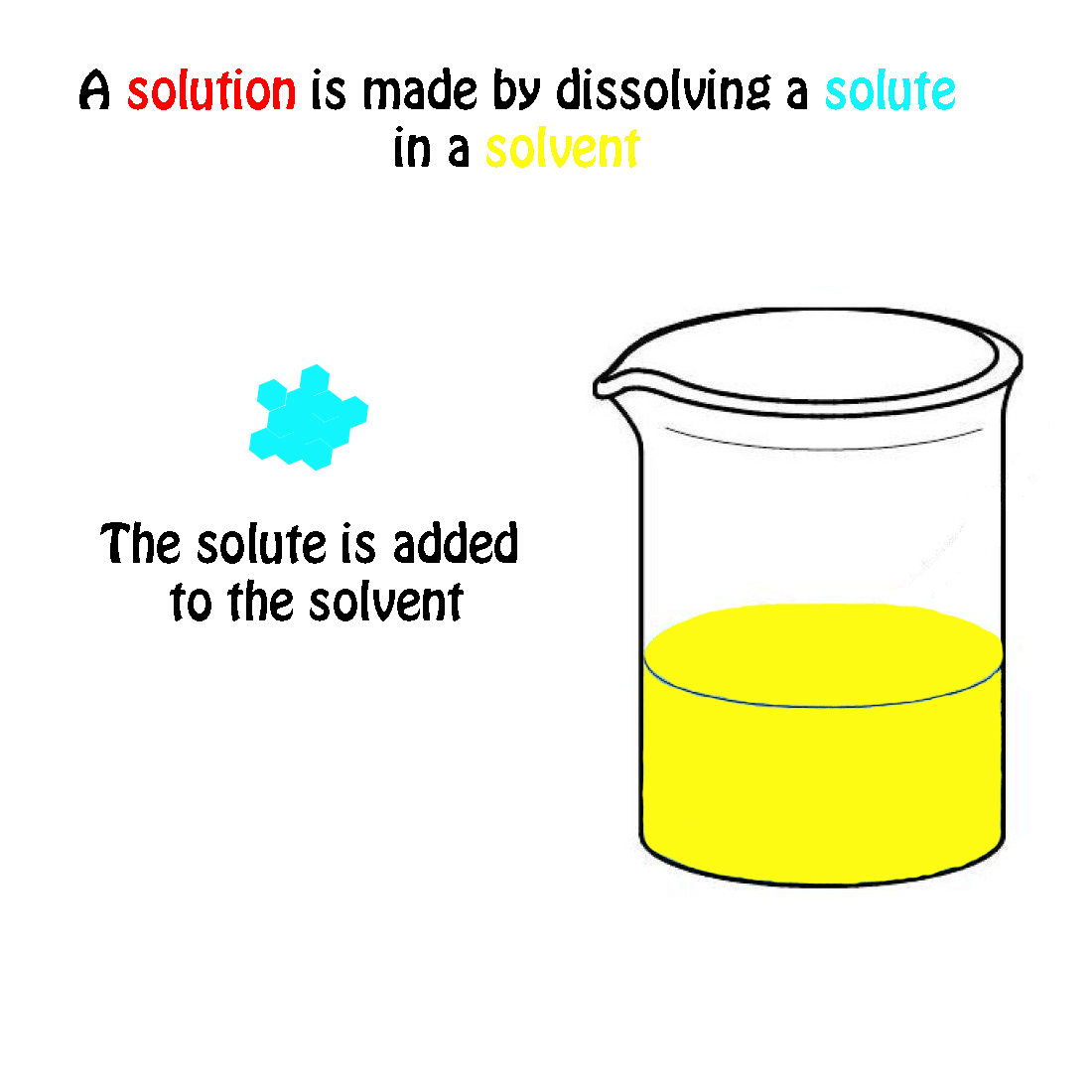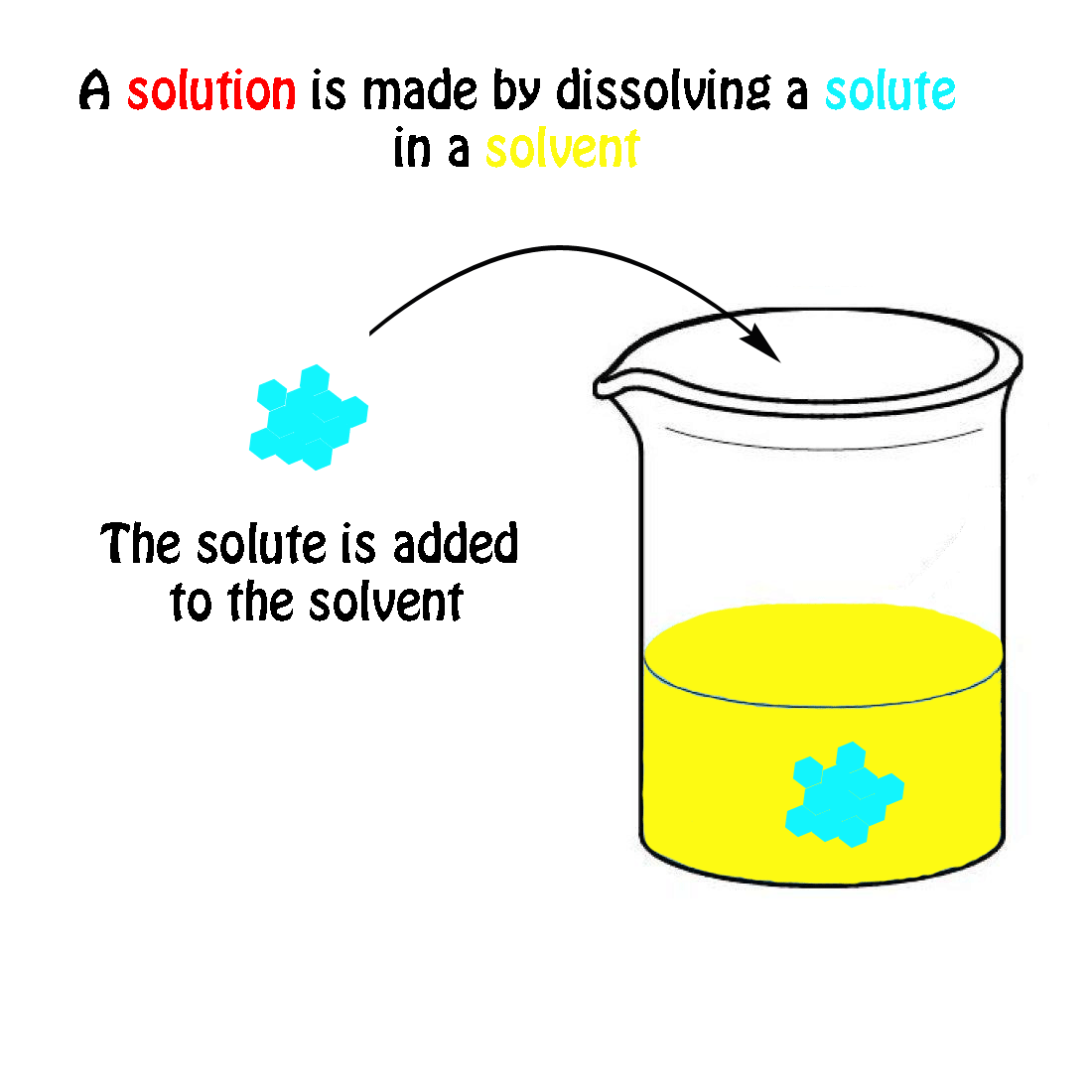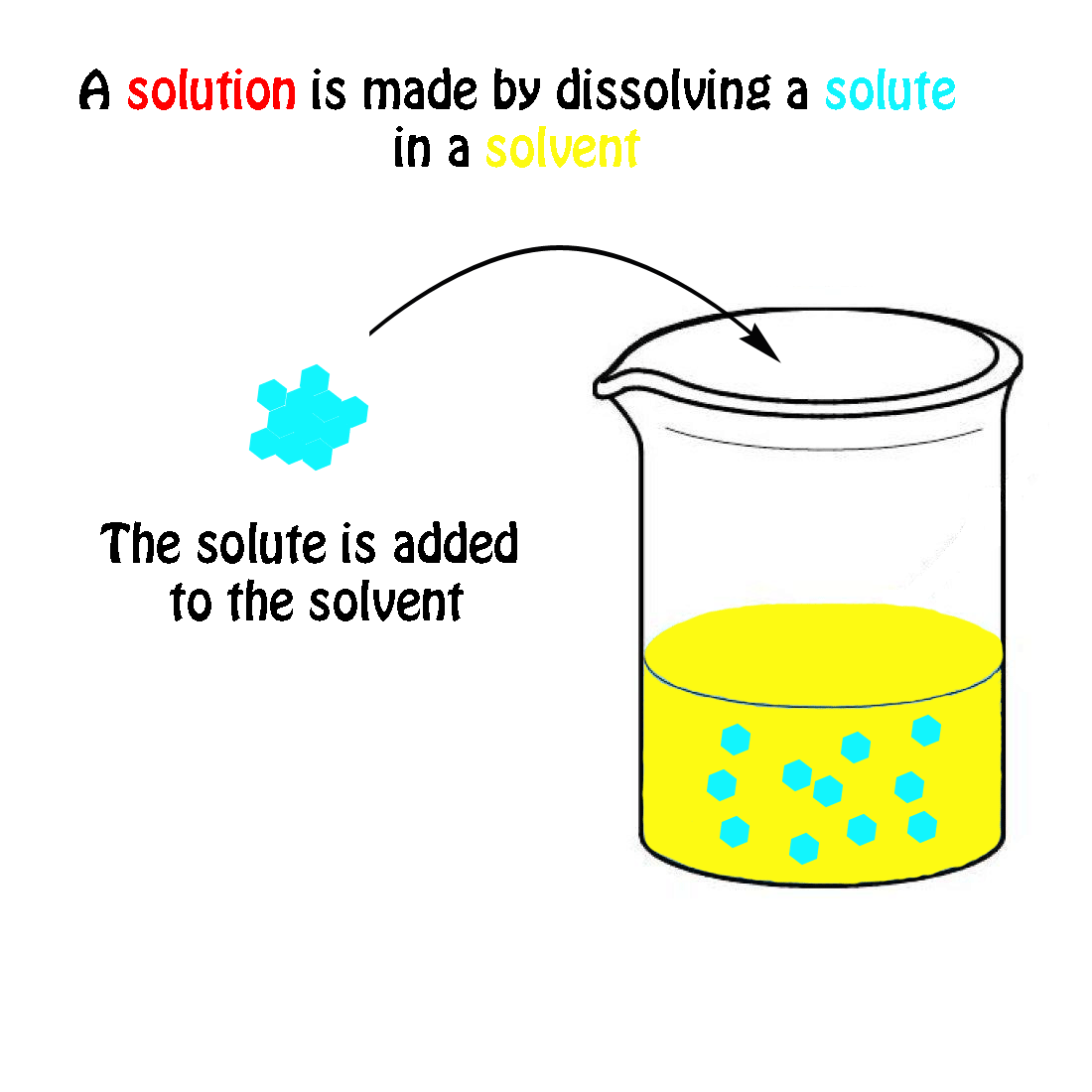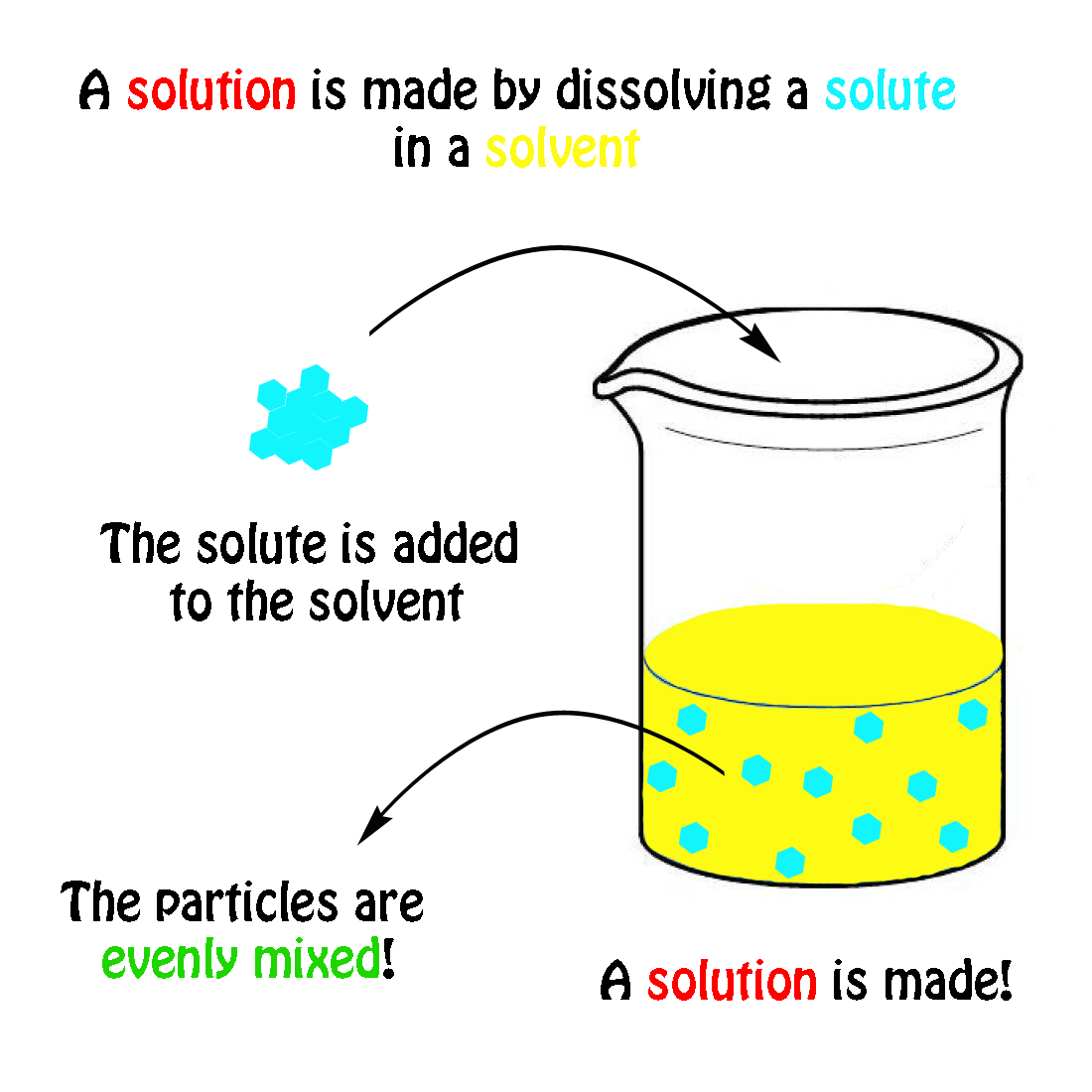
The molecular weight of NaCl is 58.44 grams/mole. If you had a 1.0

TW202144014A - Method for the preparation of an antibody drug

Lecture Topics Atomic weight, Mole, Molecular Mass, Derivation of

KENDRIYA VIDYALAYA SANGATHAN; Prepared By : ZONAL INSTITUTE OF

Doc 117 b p s xi chemistry iit jee advanced study package 2014 15
:max_bytes(150000):strip_icc()/Freezing-point-depression-58fa34d45f9b581d59c9381b.jpg)
How to Calculate Freezing Point Depression

医学基础化学( 1 ) 刘洛生CHAPTER 1 Solution ( 4h) 2 Electrolyte

Amyloid β accelerates age-related proteome-wide protein

5.85g of NaCl is dissolved in 1L of pure water. The number of ions in
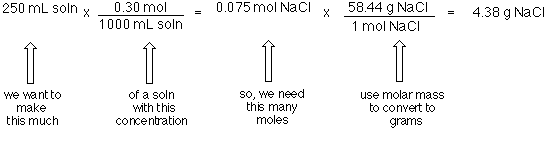
Solutions
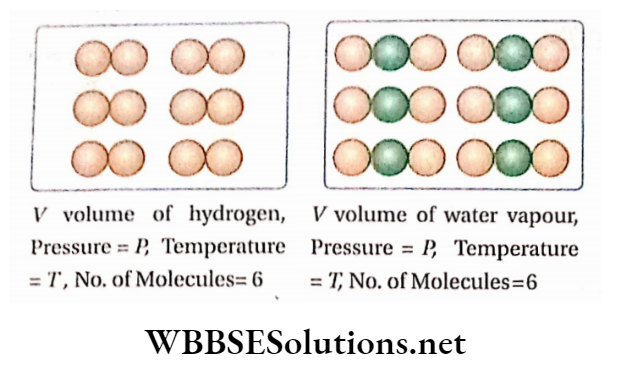
Vasantha, Author at WBBSE Solutions

The lattice energy of NaCl is -786 kJ/mol, and the enthalpy of
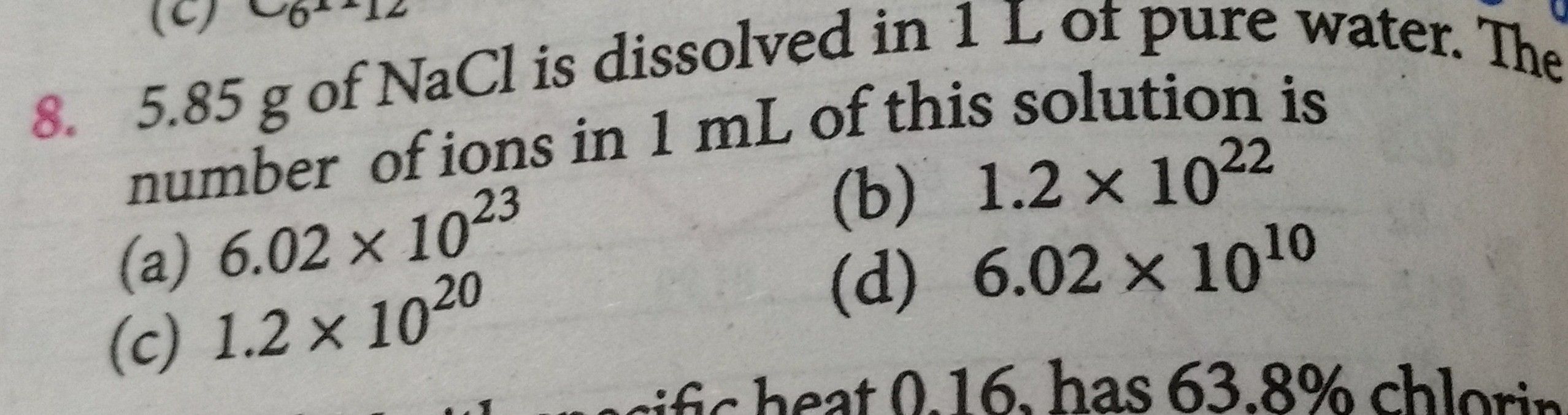
Questions and Answers of Some Basic Concepts Of Chemistry Mole









