2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology
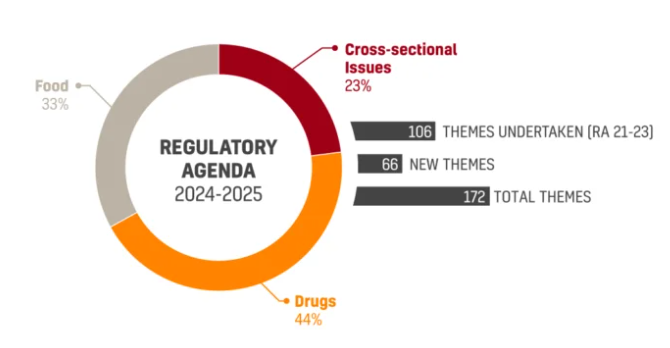
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

The Future Legal Regulation Of Artificial Intelligence In Brazil

Digital Health: Fundamentals of FDA Regulation (January 2024)

2024 IOE-BDA-DHL Group International Human Rights Conference on

Abstract Submissions - WORLDSymposium

Snapshot: procedure for design registration in Brazil - Lexology

2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA

2024 Abstract Submission

Relatório de gestão de 2022 do inpi: panorama de patentes - Lexology

Relatório de gestão de 2022 do inpi: panorama de patentes - Lexology
.jpg?rev=a681631fe4954c1a93b45372334bdc74&w=790&hash=D2DEFDB12951E171451EEE9624AC2CA9)
Celltrion's Vegzelma Becomes Fourth US-Approved Bevacizumab

FDA Regulatory News and Trends - January 19, 2024 - Lexology

Call for Presentations - 2024 Call for Presentations

Snapshot: procedure for design registration in Brazil - Lexology

BPTO publishes phase IV of the Patent Prosecution Highway (PPH

RAPS Webcast: FDA Forecast 2024: What's Next for the FDA in 2024