
Clamping astrocyte Ca 2+ in vivo with CalEx impairs vasomotion (A)
Download scientific diagram | Clamping astrocyte Ca 2+ in vivo with CalEx impairs vasomotion (A) Cartoons depicting the awake-mouse, in vivo setup whereby a trained, head-restrained animal is on a passive, air-supported Styrofoam ball (top) during imaging through a cranial window (bottom). (B) Representative image of the cranial window showing astrocyte-specific GCaMP6f fluorescence (cyan). (C) Representative image of penetrating arteriole lumen visualized via rhodamine-dextran (magenta) and astrocyte-specific GCaMP6f fluorescence (cyan). (C) Post hoc immunofluorescence images showing astrocyte GCaMP6f fluorescence (cyan) and the mCherry reporter used to visualize the CalEx construct (magenta). (D) Representative arteriole diameter traces showing vasomotor fluctuations in Tdtomato control animals (n = 12 arterioles, n = 12 animals) (red) that are disrupted in CalEx (n = 16 arterioles, n = 16 animals) (blue). (E) Summary time series of diameter traces aligned via cross-correlation showing diameter fluctuations in Tdtomato control animals (red) versus CalEx (blue). (F) Peak-to-trough diameter measurements in control animals (red) versus CalEx (blue) (unpaired t test). (G) Representative endfoot Ca 2+ traces showing Ca 2+ fluctuations in Tdtomato control animals (orange) that are disrupted in CalEx (purple). (H) Summary time series of Ca 2+ traces aligned via cross-correlation showing diameter fluctuations in Tdtomato control animals (orange) versus CalEx (purple). (I) Peak-to-trough Ca 2+ measurements in Tdtomato control animals (orange) versus CalEx (purple)(unpaired t test). (J) Frequency power analysis of arteriole diameter fluctuations (top; control in red, CalEx in blue) and endfoot Ca 2+ fluctuations (bottom; control in orange, CalEx in purple). Right panels show summary data at 0.1 Hz frequency (unpaired t test). (K and L) Cross-correlation of diameter versus endfoot Ca 2+ changes for control (K, red) and CalEx (L, blue). The inset scatterplots (bottom right) show correlation coefficients between diameter and Ca 2+ for all data points at zero time lag. All summary data are presented as mean ± SEM. * = p<0.05, ** = p<0.01, *** = p<0.001. from publication: Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback | Very-low-frequency oscillations in microvascular diameter cause fluctuations in oxygen delivery that are important for fueling the brain and for functional imaging. However, little is known about how the brain regulates ongoing oscillations in cerebral blood flow. In mouse | Arterioles, Astrocytes and Betahistine | ResearchGate, the professional network for scientists.

Intracellular diffusion of BAPTA through the astrocyte network causes

Astrocytic Ca2+ signals partake in inhibitory neurovascular coupling in a brain state-dependent manner

Calcium dynamics in astrocyte processes during neurovascular coupling

Astrocyte-specific Ca2+ activity: Mechanisms of action, experimental tools, and roles in ethanol-induced dysfunction

Astrocytes amplify cerebral blood flow elevation to sustained cortical activation in the awake mouse

Interfering with astrocytes influences neuronal activity. (A,B)
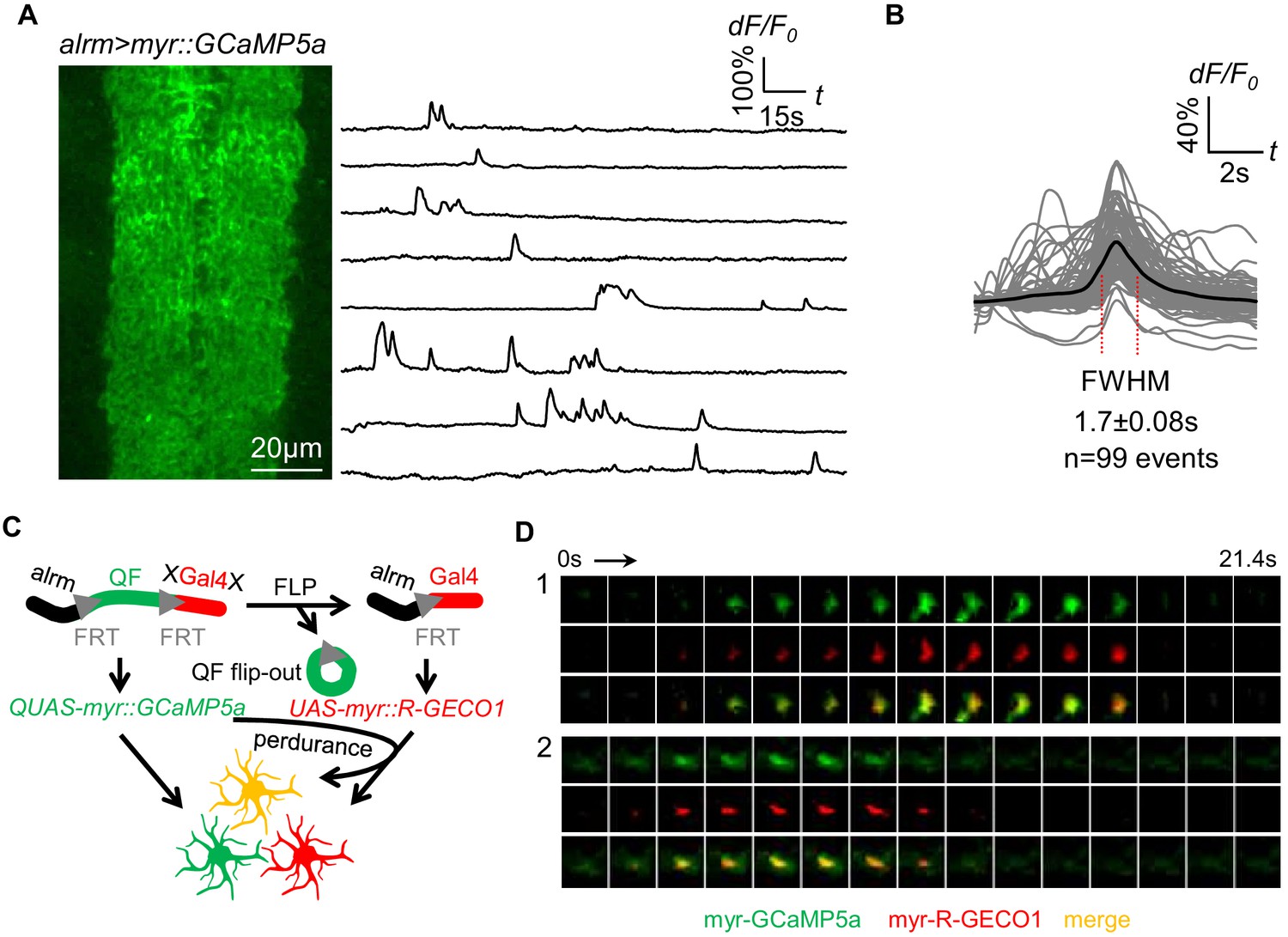
TrpML-mediated astrocyte microdomain Ca2+ transients regulate astrocyte–tracheal interactions

Local and CNS-Wide Astrocyte Intracellular Calcium Signaling Attenuation In Vivo with CalExflox Mice

Clamping astrocyte Ca 2+ in vivo with CalEx impairs vasomotion (A)

Functional hyperemia is preserved during COX-1 antagonism in awake mice

Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback - ScienceDirect

Three-dimensional Ca2+ imaging advances understanding of astrocyte biology








