Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for- CO-g- - 1-2 O2-g- CO2-g-at constant V is-67-71 K cal at 17- C- The heat of reaction at constant P at 17- C is
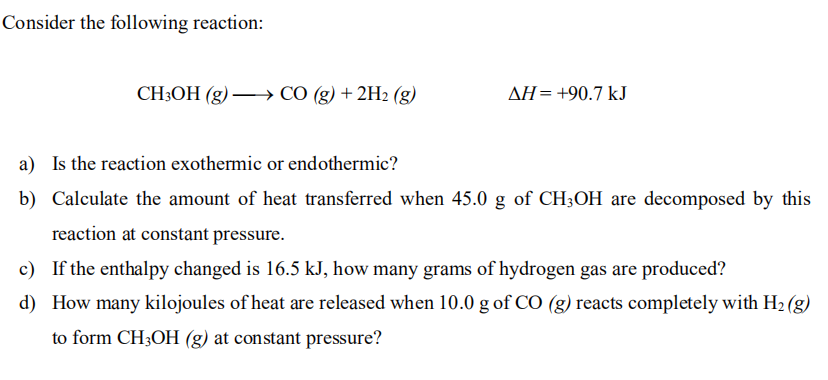
Solved Consider the following reaction: CH3OH (g) —→ CO (g)
Essential Pharma Documents: February 2017
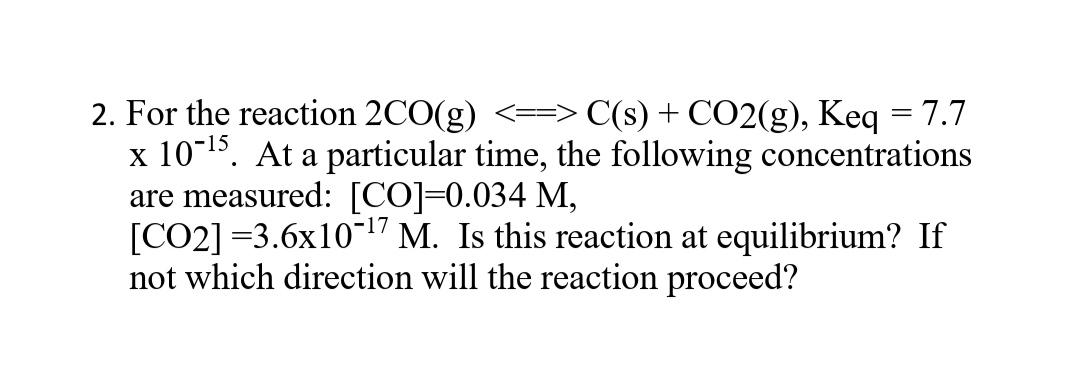
Solved For the reaction 2CO(g) => C(s) + CO2(g), Keq = 7.7 x

Thermal Decomposition of Phosgene and Diphosgene

Bioprocess Engineering Principles 2nd Edition Solutions Manual [2nd Edition, 2nd ed.] 9780122208515

heat evolved in the reaction H2 + cl2 gives to HCL is 182 kj/mole bond energy of H2,cl2 are 430 and
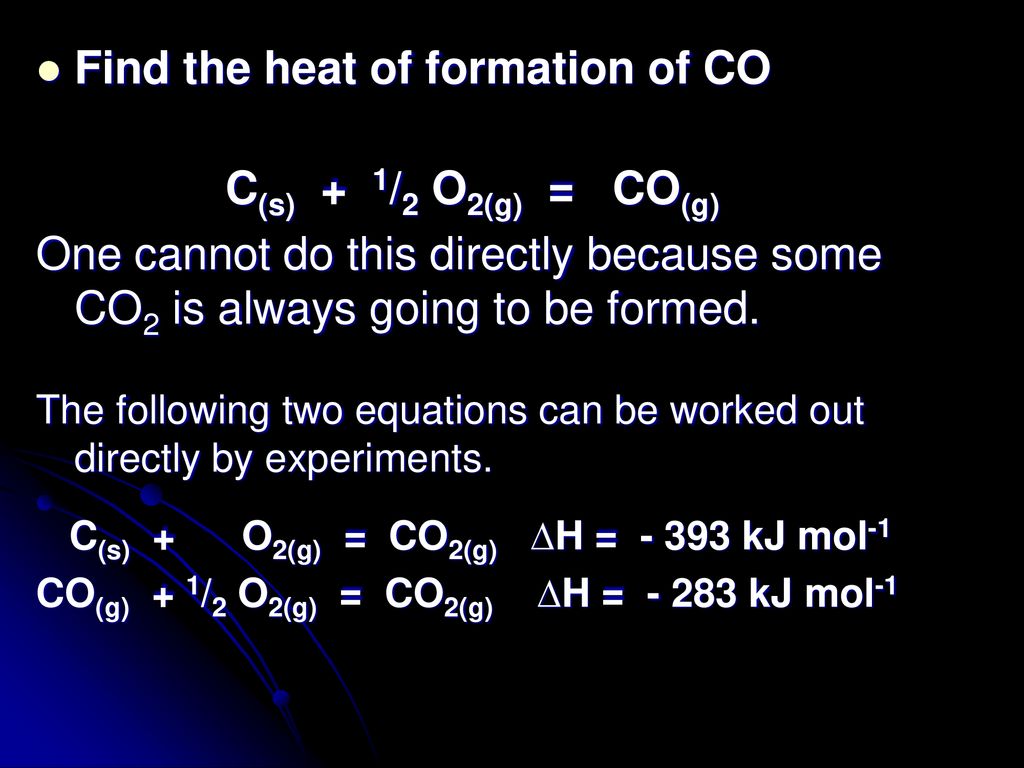
5.4.6 Hess's Law of Heat Summation [1840] - ppt download

Solved Question 27 (1 point) In the reaction, CO (g) + NO2

PDF) Chapter 8 Thermochemistry








