SalivaDirect™ COVID-19 Testing Process < Pathology
Our quick and affordable saliva-based COVID-19 test developed by Yale scientists has received FDA Emergency Use Authorization. The Pathology Clinical Molecular

SalivaDirect, Inc. (@saliva_direct) / X
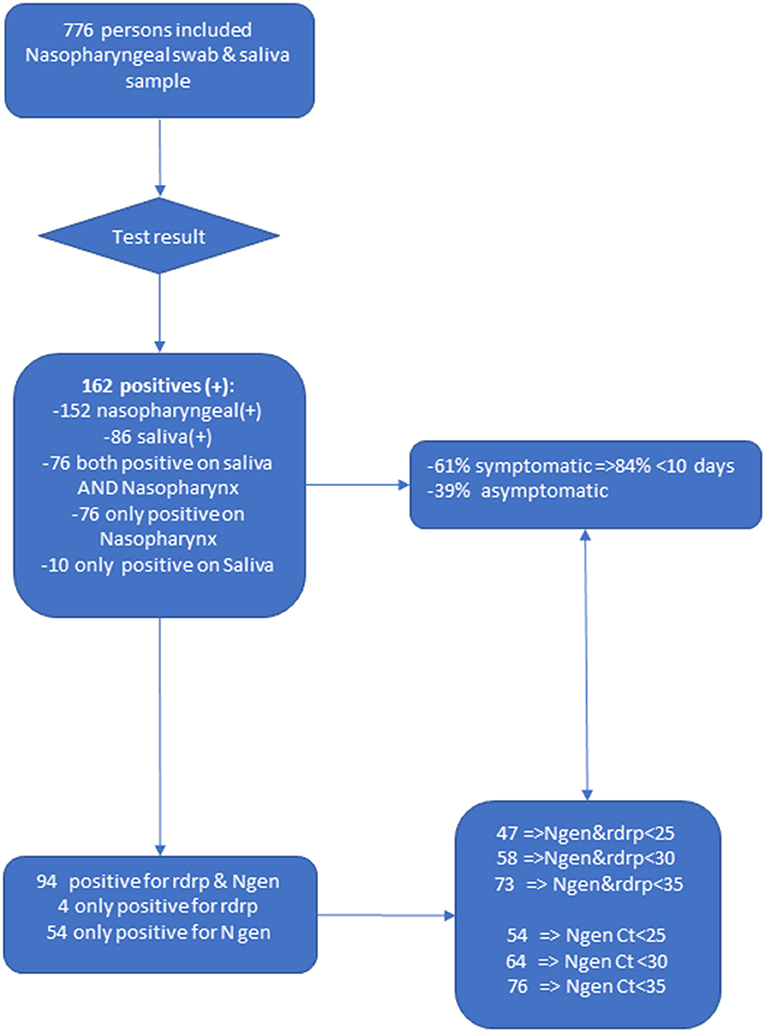
Frontiers Prospective Comparison of Saliva and Nasopharyngeal Swab Sampling for Mass Screening for COVID-19
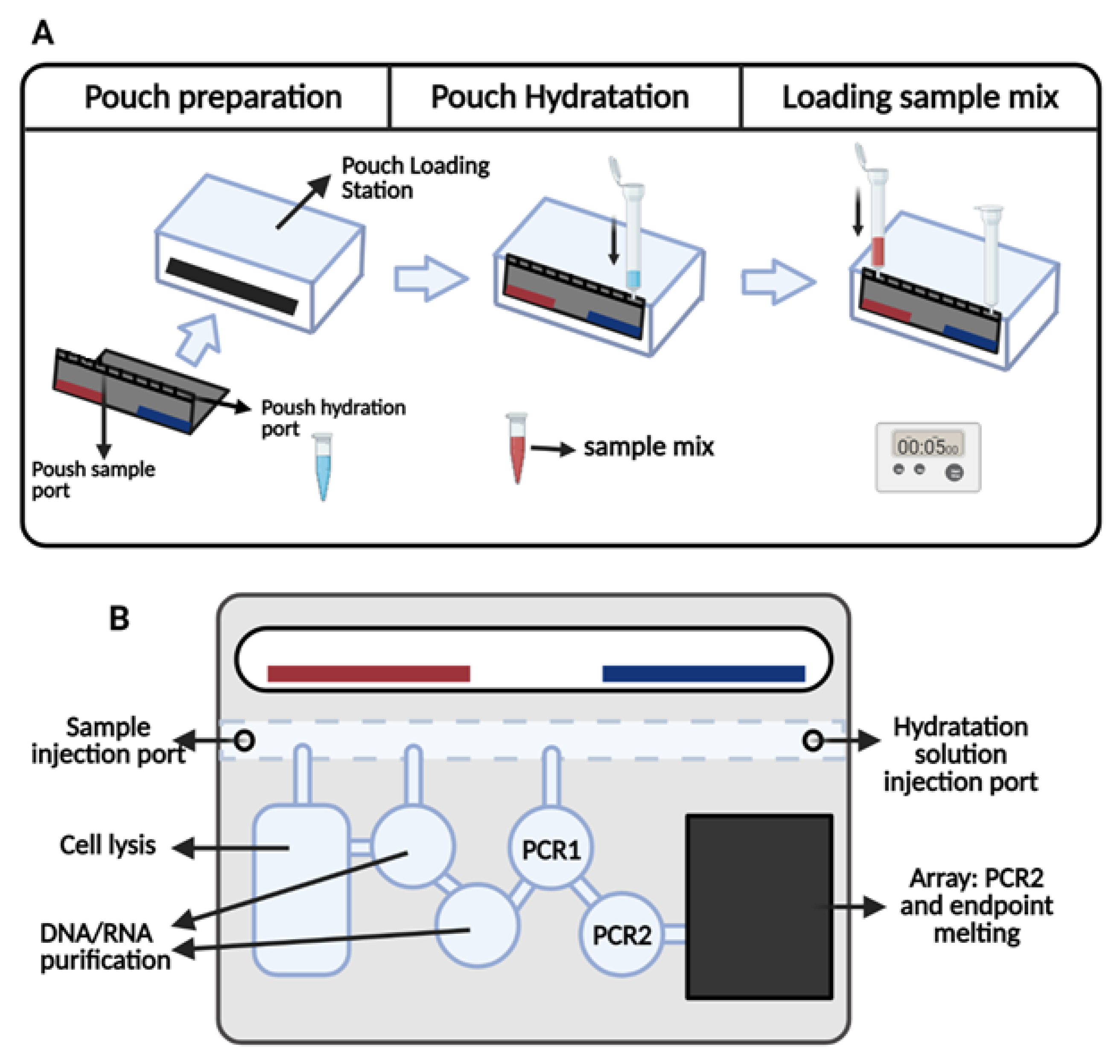
Bioengineering, Free Full-Text

COVID Test Direct Diagnostics

SaliVISION: a rapid saliva-based COVID-19 screening and diagnostic test with high sensitivity and specificity

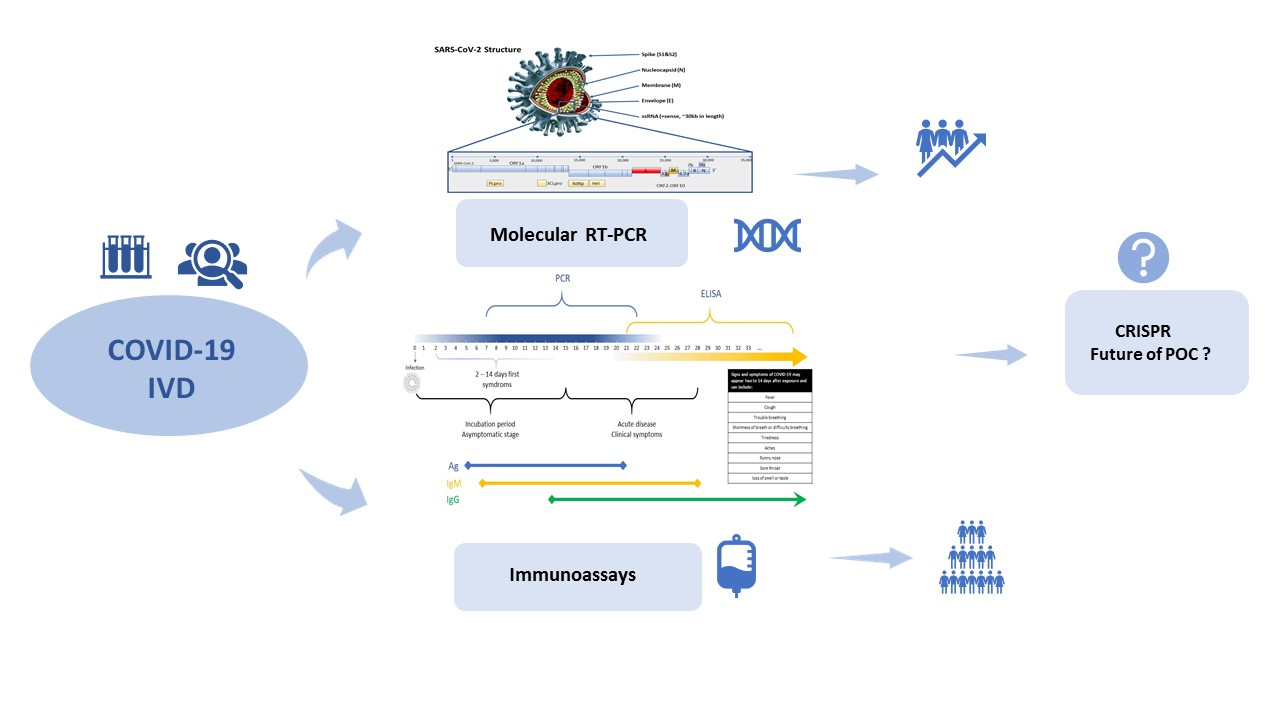
Antigen and Molecular Tests for COVID-19
FDA Clears First Home Saliva Test for Coronavirus

A multiplexed, paired-pooled droplet digital PCR assay for detection of SARS-CoV-2 in saliva

About SalivaDirect < SalivaDirect™
Diagnostics, Free Full-Text

Development and Implementation of a Simple and Rapid Extraction-Free Saliva SARS-CoV-2 RT-LAMP Workflow for Workplace Surveillance