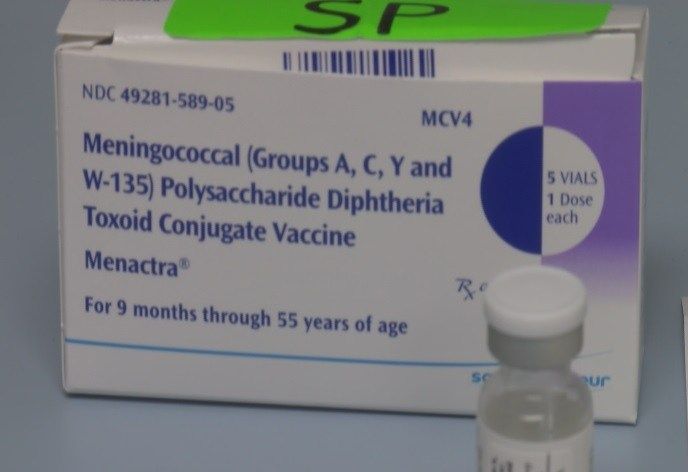
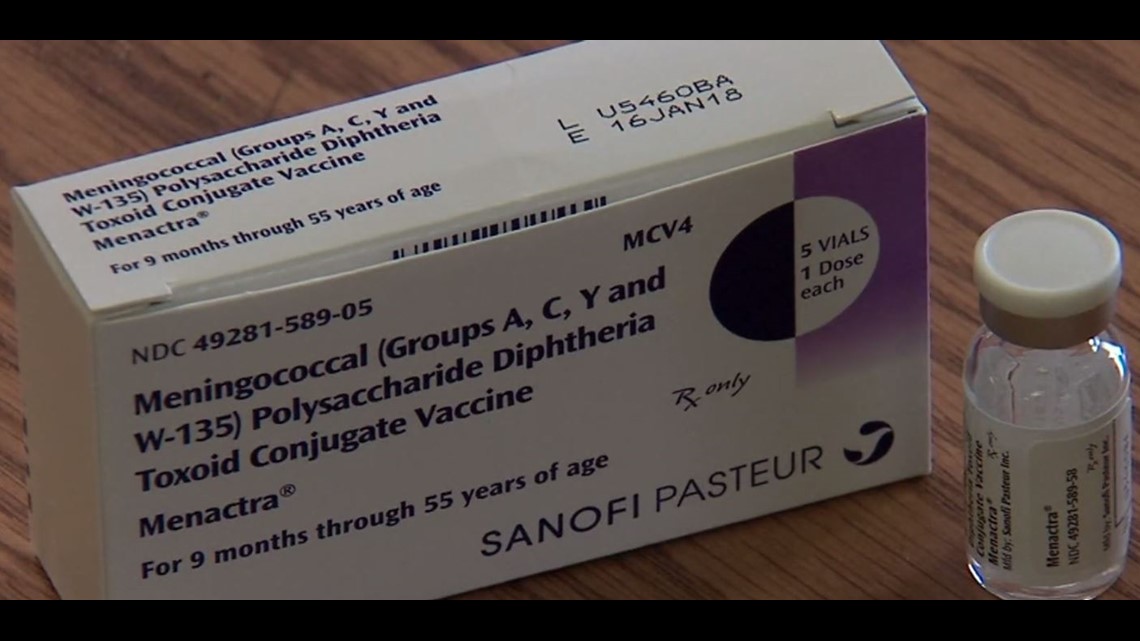
Sanofi Pasteur Announces FDA Approval of Menactra Meningococcal Conjugate Vaccine Indication for Infants
/PRNewswire/ -- Sanofi Pasteur, the vaccines division of the sanofi-aventis Group (EURONEXT: SAN and NYSE: SNY), announced today that the U.S. Food and Drug
Menactra Full Prescribing Information, Dosage & Side Effects
Meningococcal and Meningococcal Vaccine Information - National Vaccine Information Center (NVIC)
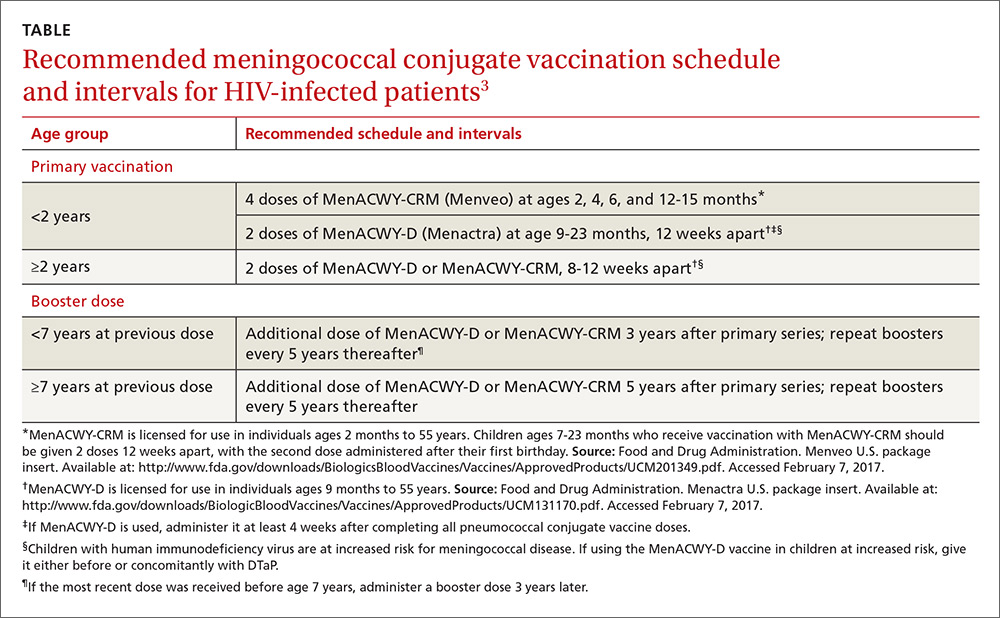
ACIP vaccine update, 2017
The meningococcal conjugate vaccine: Uses, Side Effects, Dosage & Reviews

PDF] Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020

Sanofi Pasteur 589-05 - McKesson Medical-Surgical

MENACTRA 4 - Meningococcal 4mcg Polysaccharide Diphtheria Toxoid Conjugate Vaccine at Rs 4950.00/box, Afzal Gunj, Hyderabad

Sanofi Pasteur announces FDA approval of Menactra® - European Pharmaceutical Review
The meningococcal conjugate vaccine: Uses, Side Effects, Dosage & Reviews

PDF) Meningococcal Quadrivalent Tetanus Toxoid Conjugate Vaccine (MenACWY-TT, Nimenrix™): A review of its Immunogenicity, Safety, Co-Administration, and Antibody Persistence

Safety and Immunogenicity of a Meningococcal Quadrivalent Conjugate Vaccine in Five- to Eight-Year-Old Saudi Arabian Children Previously Vaccinated with Two Doses of a Meningococcal Quadrivalent Polysaccharide Vaccine

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health

The status of COVID-19 vaccines in India: A review