Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Vaccines, Free Full-Text
PDF) Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Full article: Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age
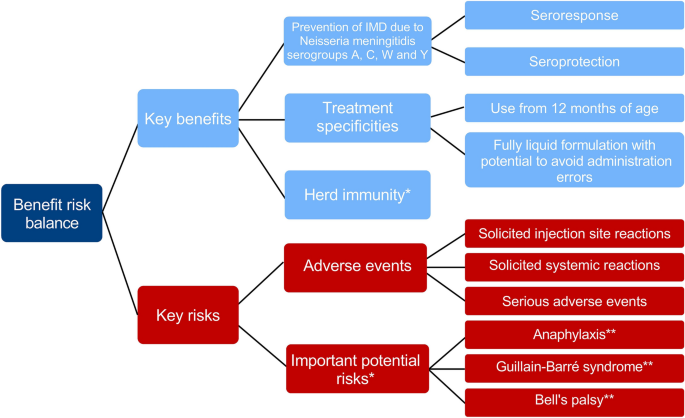
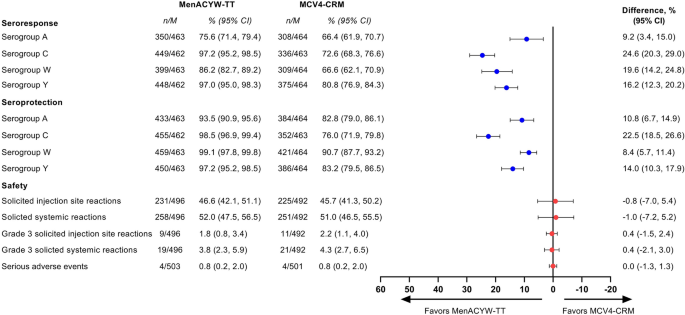
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older
Microorganisms, Free Full-Text
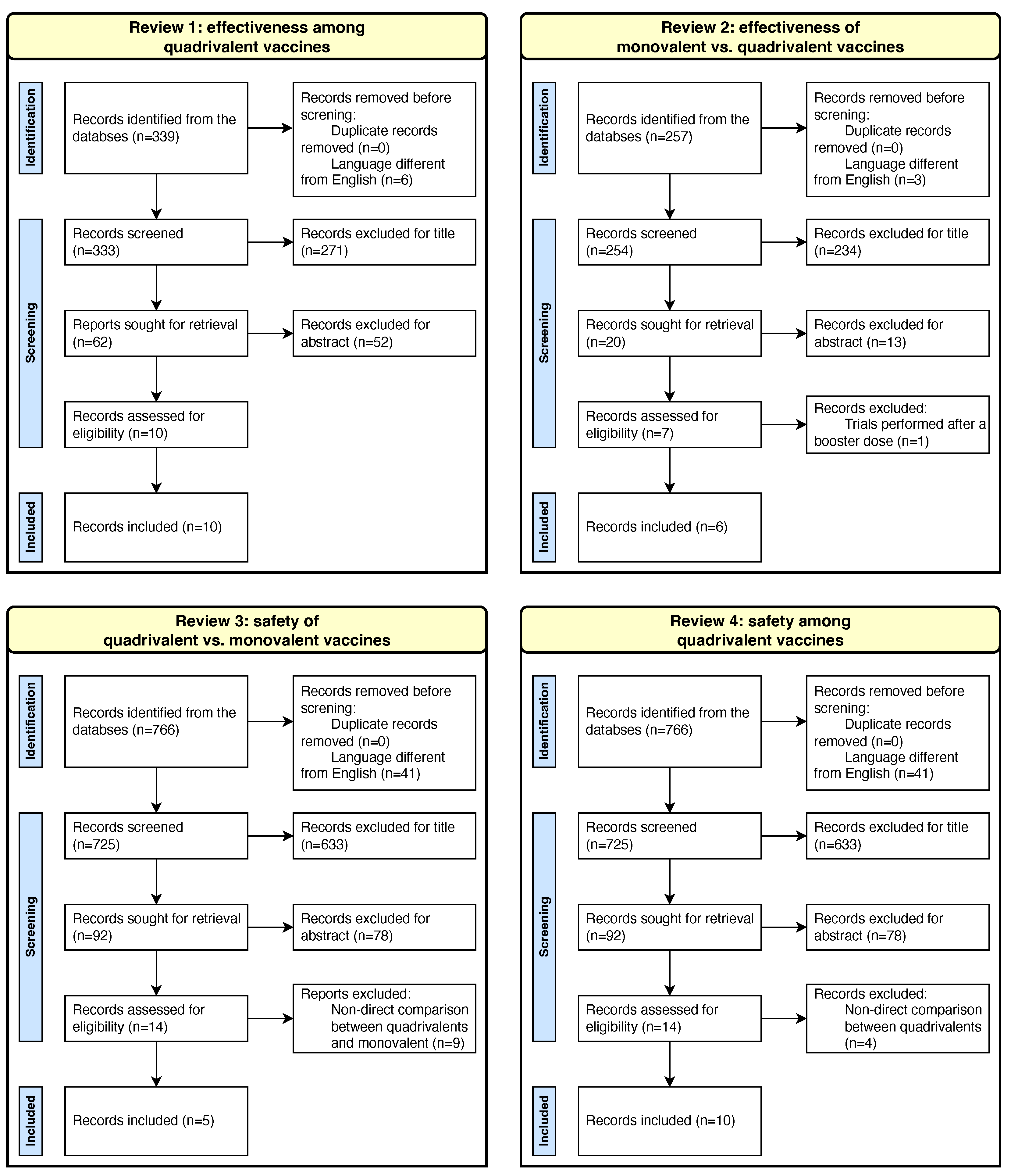
Vaccines, Free Full-Text

Full article: Real-world impact and effectiveness of MenACWY-TT
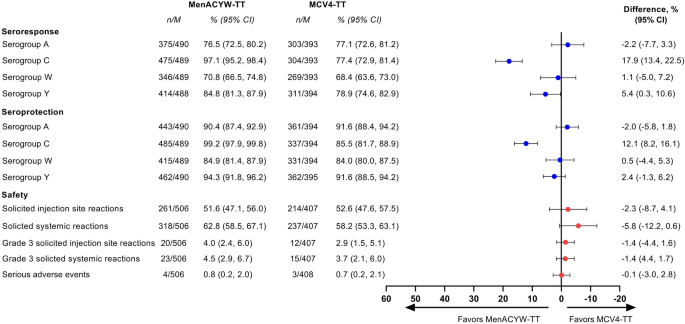
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Prevention of Meningococcal Infection in the United States: Current Recommendations and Future Considerations - ScienceDirect

Full article: Potential public health impact of a Neisseria meningitidis A, B, C, W, and Y pentavalent vaccine in the United States

Meningococcal Vaccine: Most Up-to-Date Encyclopedia, News & Reviews

Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence

Immunogenicity and impact on nasopharyngeal carriage of a single dose of PCV10 given to vietnamese children at 18 months of age - The Lancet Regional Health – Western Pacific