
Update on REMS-Required Testing During COVID-19 Pandemic - MPR
“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

The Risk Evaluation and Mitigation Strategy (REMS) Public Dashboard: Improving Transparency of Regulatory Activities

Federal Register :: Medicare Program; Contract Year 2025 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for

Medicina, Free Full-Text

FDA Relaxes Certain Lab and Imaging Test Requirements for REMS Programs During the COVID-19 Pandemic

COVID-19 Resource Center - Texas Hospital Association

Risk Evaluation and Mitigation Strategy programs: How they can be improved
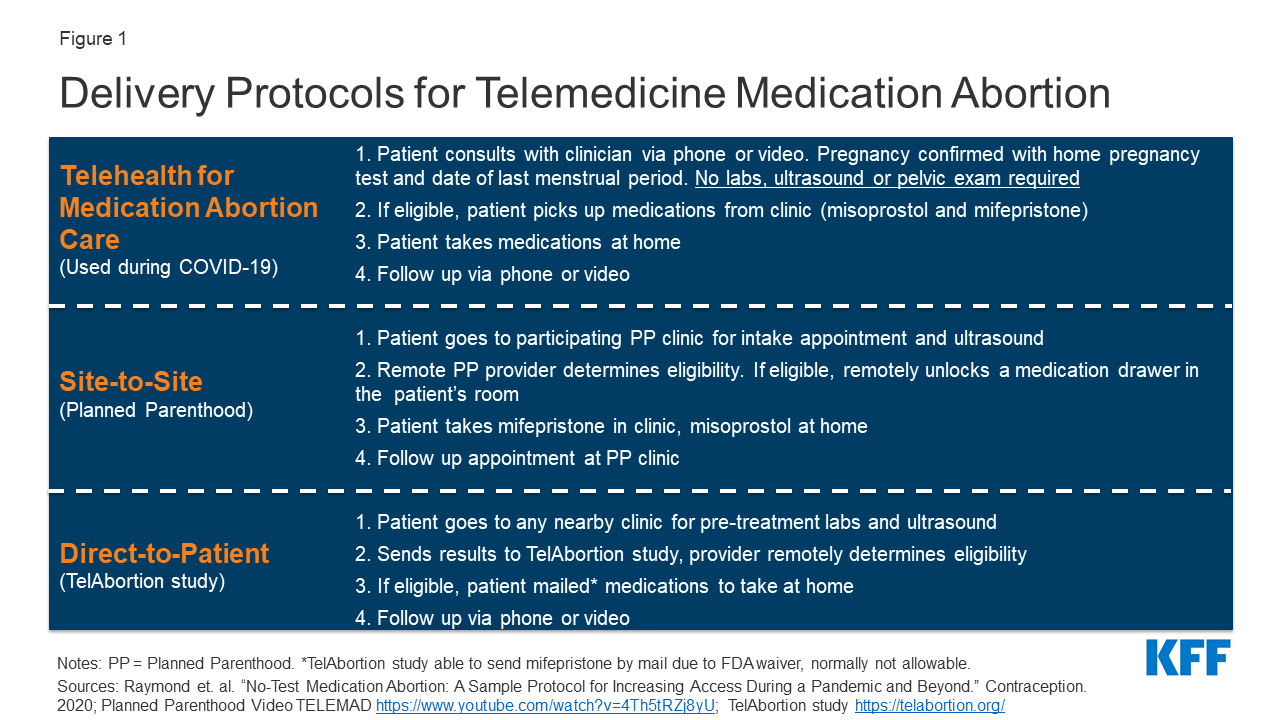
Medication Abortion and Telemedicine: Innovations and Barriers During the COVID-19 Emergency

COVID-19 Resource Center - Texas Hospital Association

COVID-19: Federal Efforts Could Be Strengthened by Timely and Concerted Actions
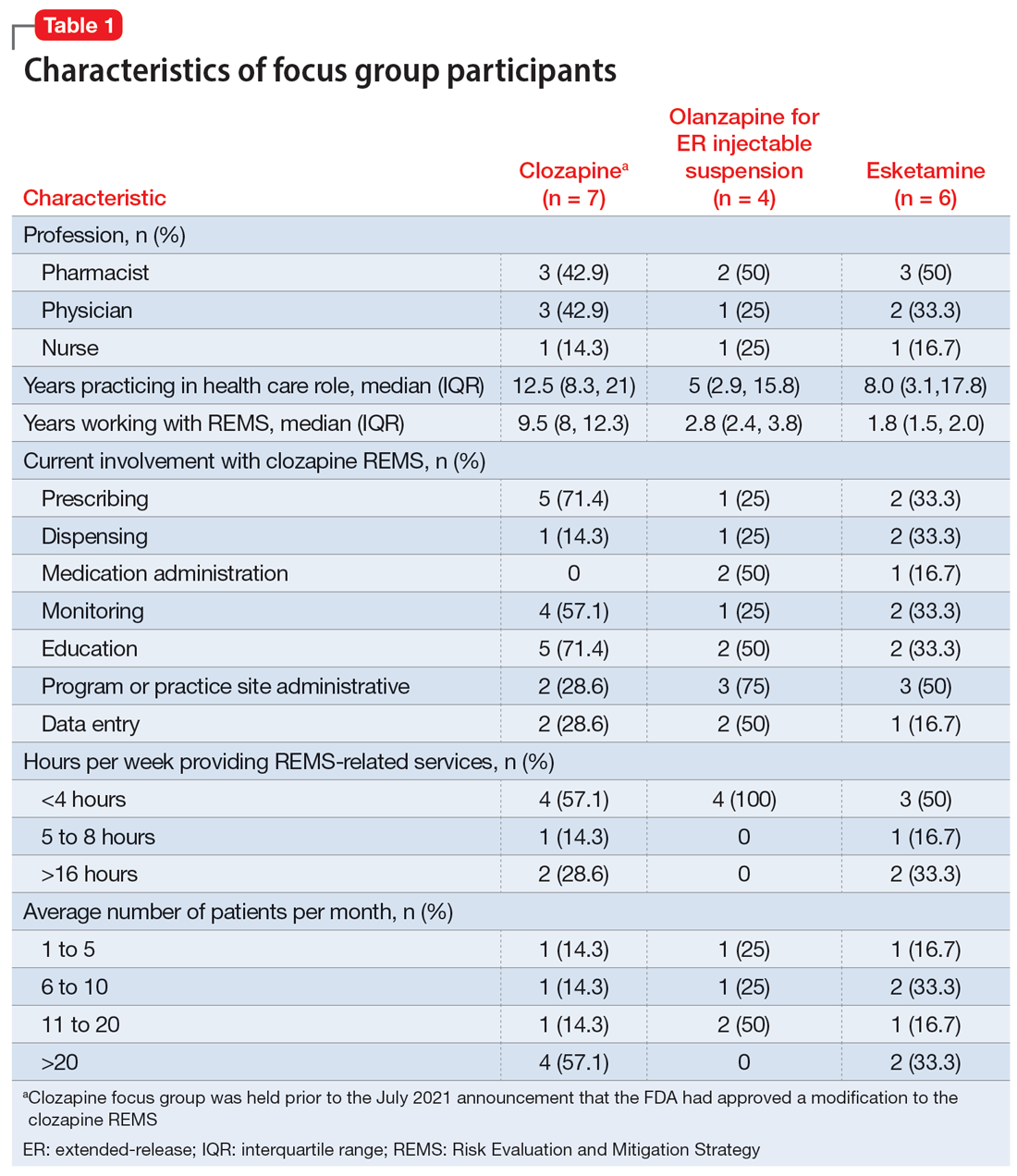
Risk Evaluation and Mitigation Strategy programs: How they can be improved

The Risk Evaluation and Mitigation Strategy (REMS) Public Dashboard: Improving Transparency of Regulatory Activities

Update on REMS-Required Testing During COVID-19 Pandemic - MPR
Full article: Improving clozapine utilization will require continued advocacy, drug sponsor interest, and FDA support to address REMS issues









