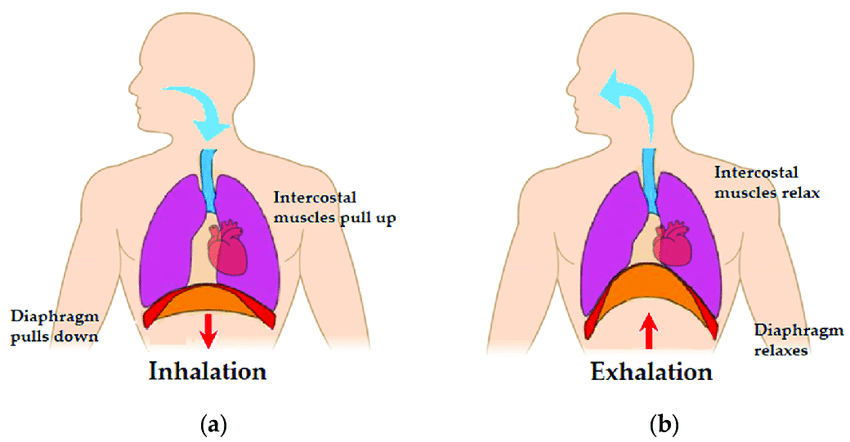
Post-inhalation cough with therapeutic aerosols: Formulation considerations - ScienceDirect

The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity - The Lancet Respiratory Medicine

PDF) In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals
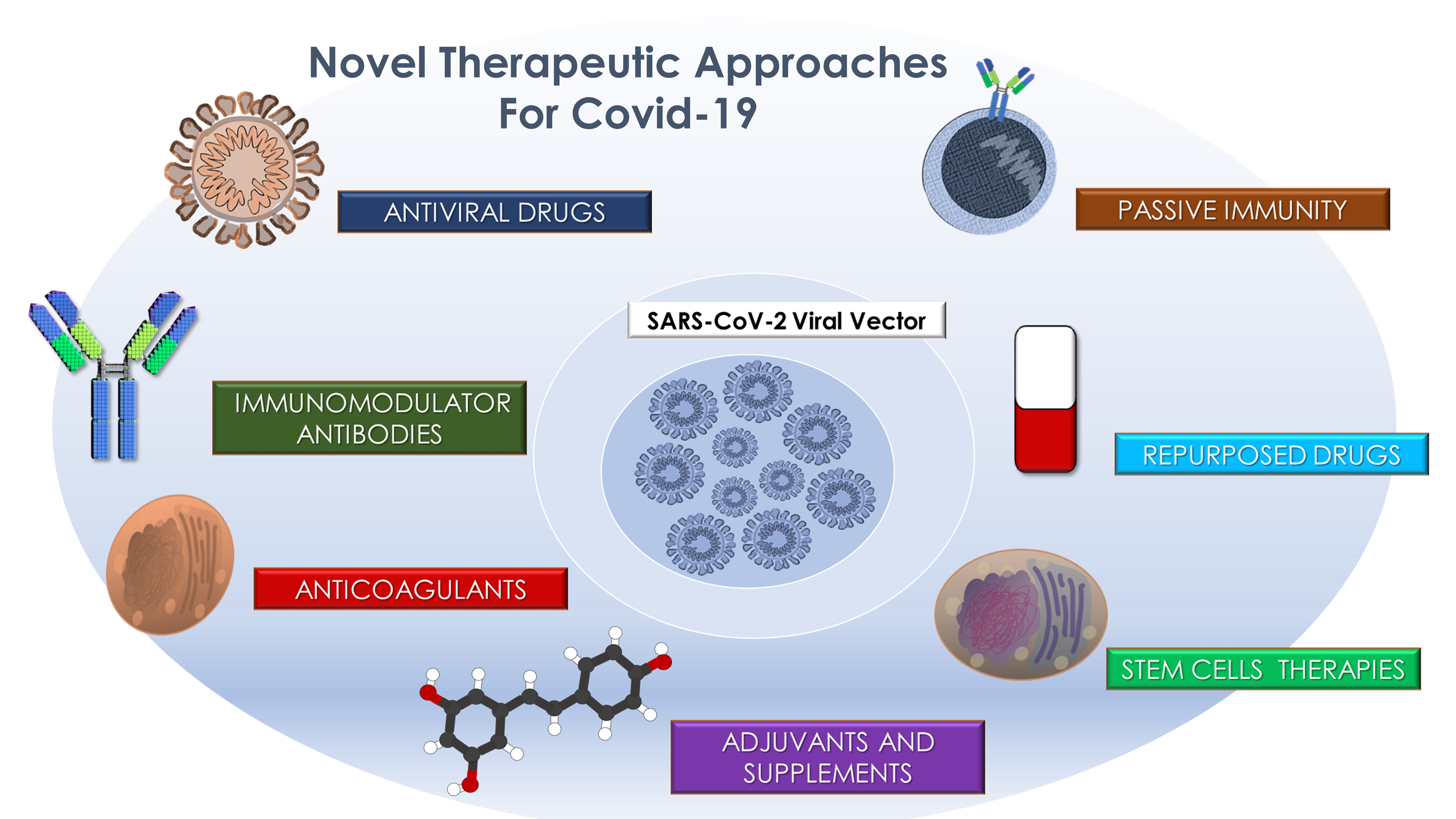
Antioxidants, Free Full-Text

Regulatory pitfalls and opportunities when repurposing for inhalation therapy - ScienceDirect

Pharmacokinetic Considerations for Optimizing Inhaled Spray-Dried Pyrazinoic Acid Formulations

Inhaled therapy for COVID-19: Considerations of drugs, formulations and devices - ScienceDirect

PDF) Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study

Modeling of inhaled corticosteroids delivery for topical croup treatment in pediatric upper airways - ScienceDirect

Post-inhalation cough with therapeutic aerosols: Formulation considerations - ScienceDirect

PDF) Effect of a spacer on total systemic and lung bioavailability in healthy volunteers and in vitro performance of the Symbicort ® (budesonide/formoterol) pressurized metered dose inhaler