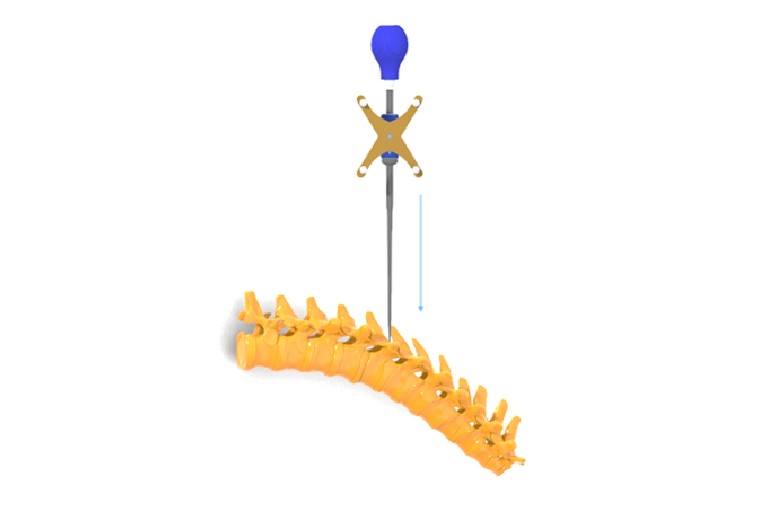
CTL Amedica granted FDA 510(k) approval for Navigation Instrument
4.6
(162)
Écrire un avis
Plus
€ 28.00
En Stock
Description

Medical Device Testing Requirements for 510(k) Submissions - In

IFU for Medical Devices, a Definitive Guide (EU & US)
510k Medical Devices Premarket Notification - Eclevar

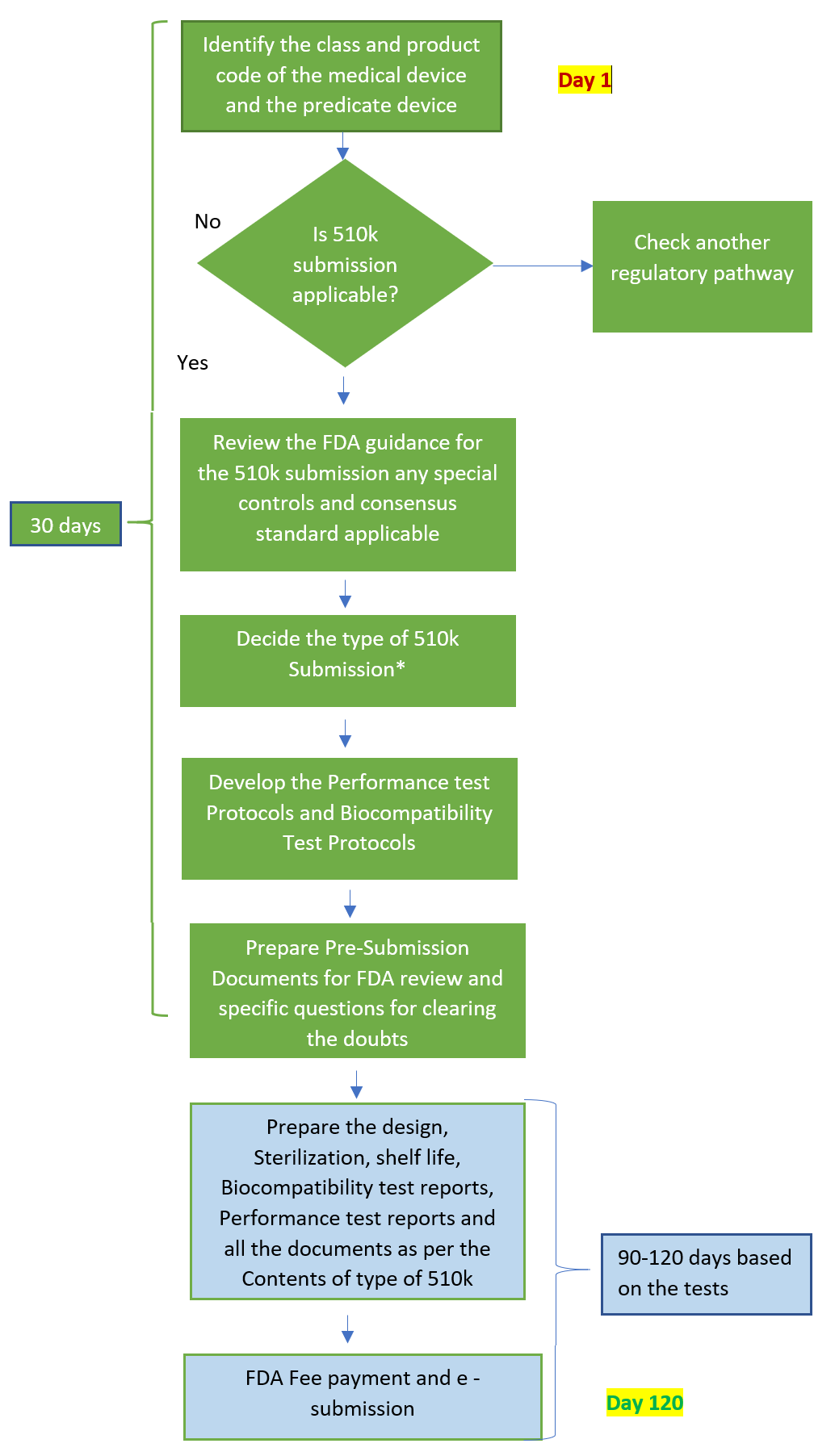
Getting Clearance for Your Medical Device - 510(k) Flowchart Included

CTL Amedica on LinkedIn: CTL Amedica is proud to announce FDA 510
Best FDA 510k Consultants For Medical Device - I3CGLOBAL
%20predicate%20device.png?width=521&height=351&name=510(k)%20predicate%20device.png)
Everything you need to know about the FDA 510(k) submission

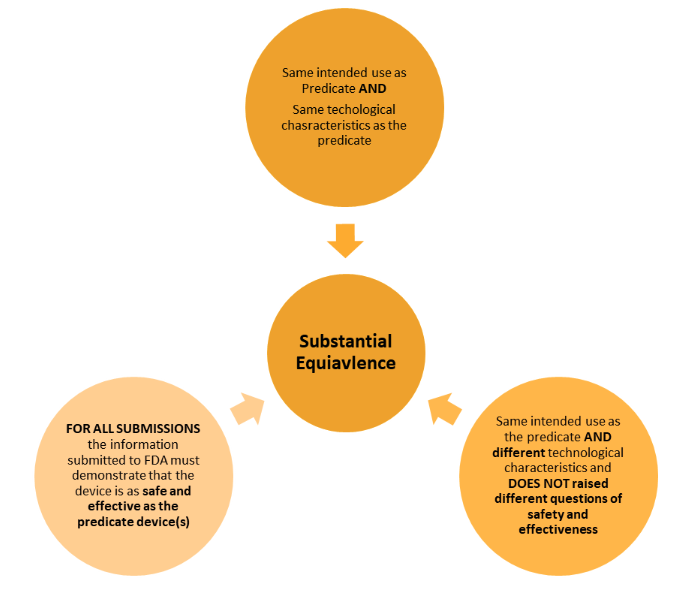
PDF) A Review on Substantial Equivalence of Medical Devices- USFDA

USA Overview of the Medical Device Market (video)
PreparationSubmission-Cover.webp?width=267&height=348&name=fda-CaseStudy-Guiding510(k)PreparationSubmission-Cover.webp)
FDA 510(k) Explained: A Basic Guide to Premarket Notification
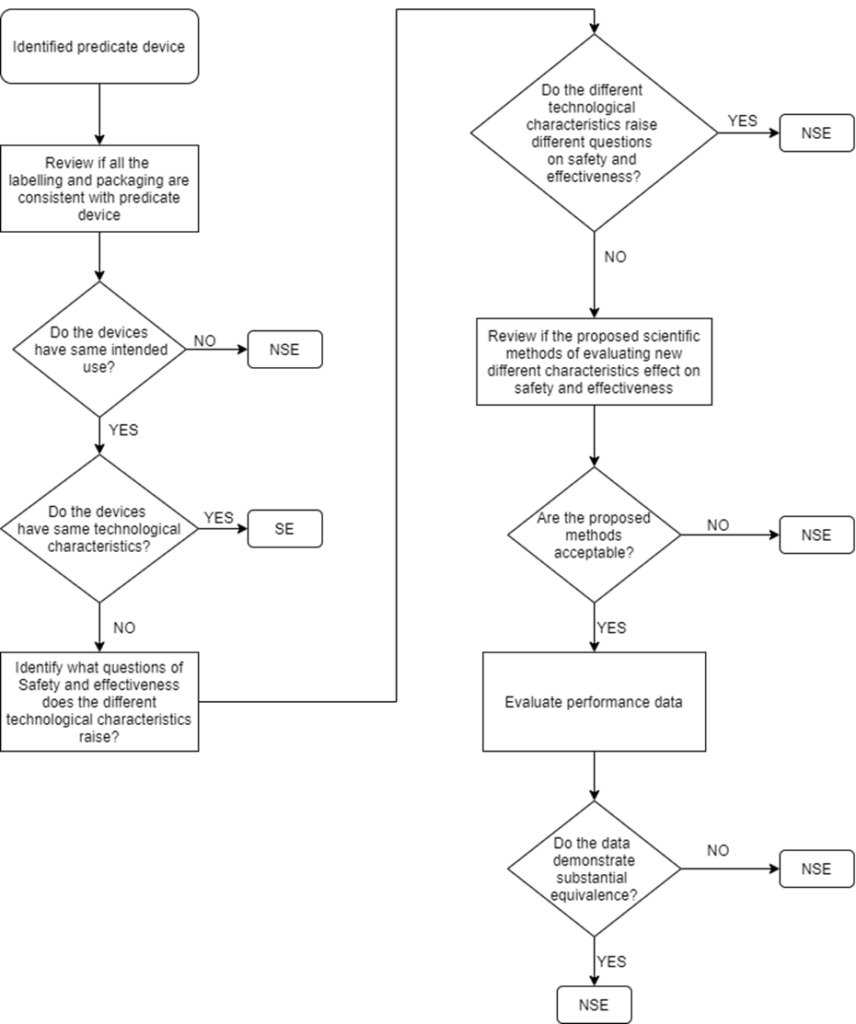
FDA's 510(K) Submission Process

Recent FDA 510(k) Clearances in Spine
Tu pourrais aussi aimer









